44 fda misleading food labels
CFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (6) FDA means the Food and Drug Administration. (7) Heading means the required statements in quotation marks listed in paragraphs (c)(2) through (c)(9) of this section, excluding subheadings (as defined in paragraph (a)(9) of this section). (8) Inactive ingredient means any component other than an active ingredient. Regulation of the U.S. Food Processing Sector — Food Law The U.S. food processing sector is extensively regulated by state and federal agencies. Federal agencies dominate the regulatory oversight: USDA FSIS for the meat and poultry processing businesses and FDA for all other food processing businesses. State agencies also have an active role in overseeing food processing businesses within their respective states, but their role is in …
Small Businesses & Homemade Cosmetics: Fact Sheet | FDA Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA (1-888-463-6332)
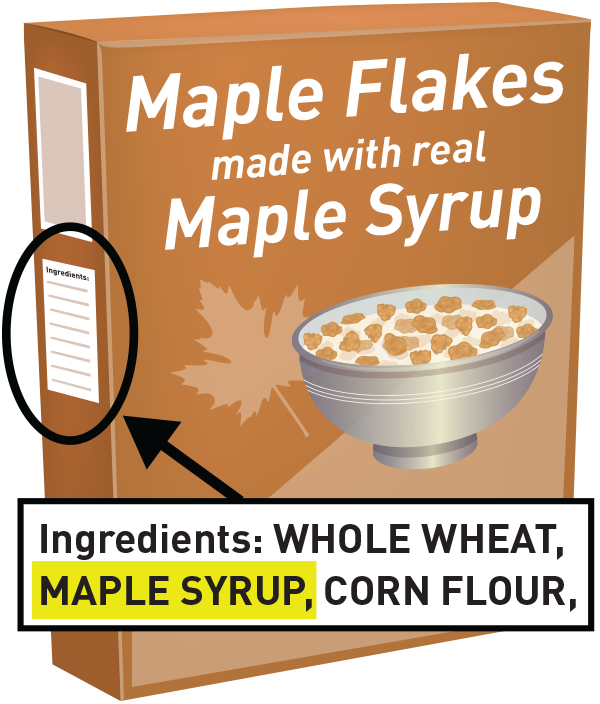
Fda misleading food labels
13 Misleading Food Label Claims and How Not to Be Tricked Oct 20, 2021 · The Food and Drug Administration (FDA) provides guidelines for a variety of common food labels, including sugar-free. While the term suggests that products labeled this way would be completely free of sugar, they can actually contain up to 0.5 grams of sugar in a single serving size. FDA Compliant Nutrition Labels | Food Lab - NUTRITIONAL … FDA-compliant nutrition labels help you avoid misleading statements and claims to assure your food label artwork conveys truthful messaging. Along with preventing avoidable customer injury and potential litigation, compliant food labeling supports customers who are searching for the best food products for their families. How FDA Failures Contributed to the Opioid Crisis To finally end the opioid crisis, the FDA must enforce the Food, Drug, and Cosmetic Act, and it must act on recommendations from the NAS for an overhaul of its opioid approval and removal policies. The broad indication on opioid labels must be narrowed, and an explicit warning against long-term use and high-dose prescribing should be added.
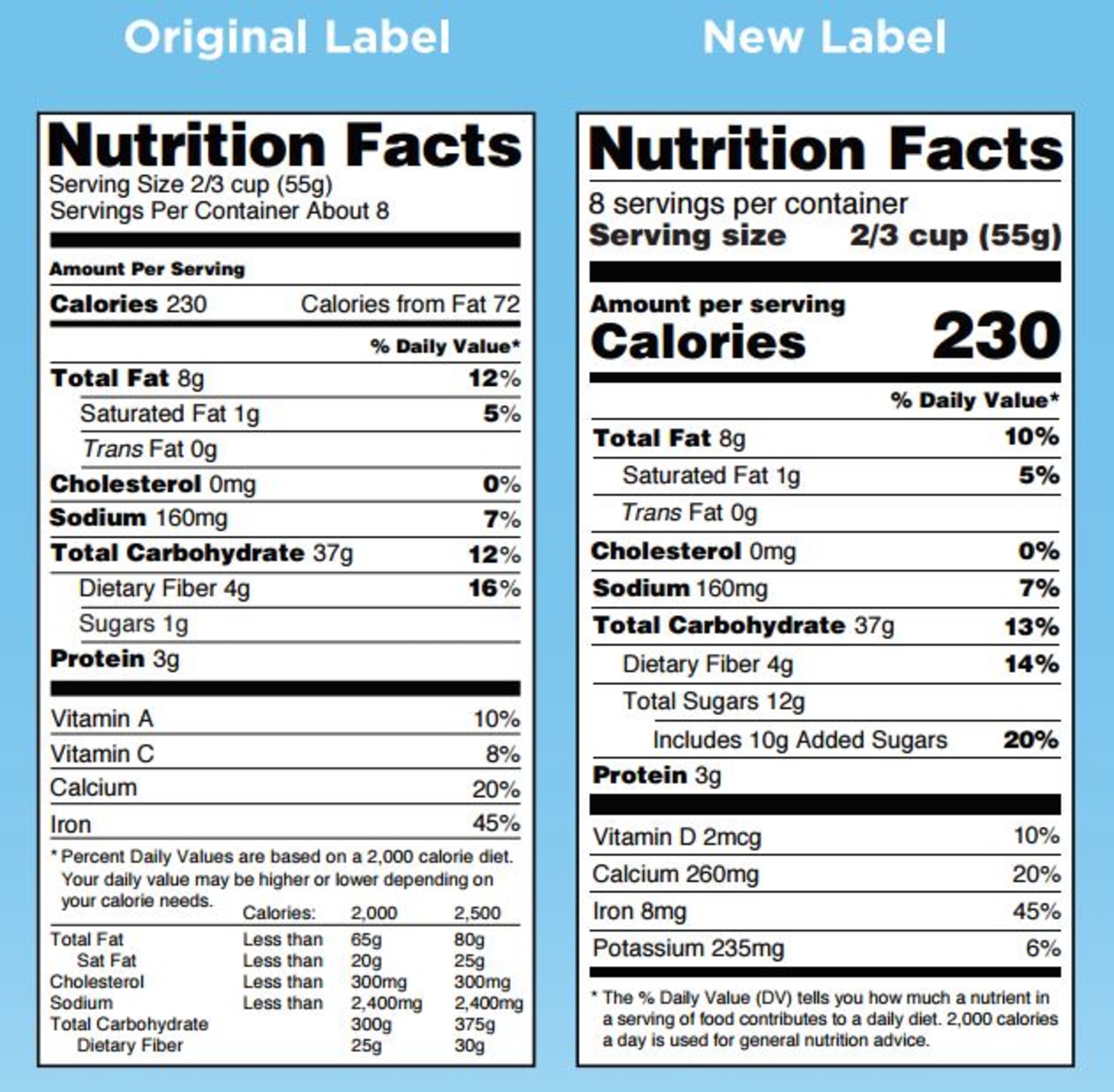
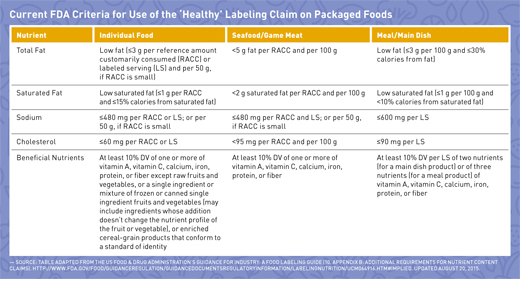
Fda misleading food labels. Guidance for Industry: Questions and Answers Regarding Food ... For questions regarding this document, contact Carol D'lima at the Center for Food Safety and Applied Nutrition (CFSAN) at 301-436-2371 (Updated phone: 240-402-1697) or Carol.Dlima@fda.hhs.gov ... Industry Resources on the Changes to the Nutrition Facts Label Sugar content claims described in 21 CFR 101.60(c), such as “sugar free” and “no sugar,” are required to be accompanied by a statement that the food is “not a reduced calorie food ... Label Claims for Conventional Foods and Dietary Supplements | FDA Mar 07, 2022 · There are three ways in which FDA exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990 ... Food Labeling & Nutrition | FDA May 16, 2022 · What's new in food labeling and nutrition, including label claims, nutrition labeling for restaurants, and links to industry guidance.
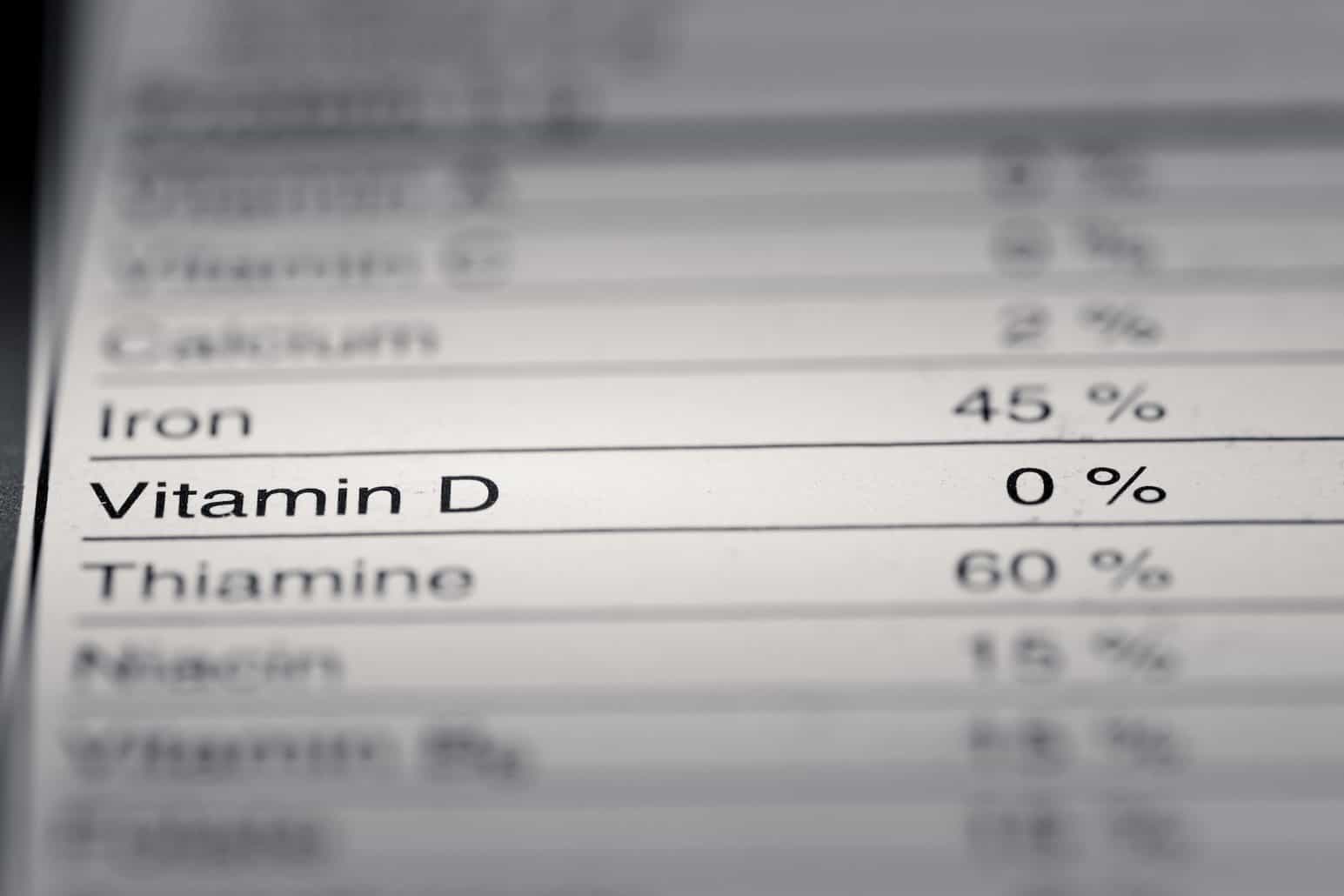
Gluten and Food Labeling | FDA - U.S. Food and Drug Administration Since 2014, the U.S. Food and Drug Administration (FDA) has required that claims on food labels that a food contains no gluten meet a clear standard that assures consumers that “gluten-free ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Jul 20, 2022 · The requirement for inclusion of the ZIP code shall apply only to consumer commodity labels developed or revised after the effective date of this section. In the case of nonconsumer packages, the ZIP code shall appear either on the label or the labeling (including invoice). ... packs, or distributes a food at a place other than his principal ... How FDA Failures Contributed to the Opioid Crisis To finally end the opioid crisis, the FDA must enforce the Food, Drug, and Cosmetic Act, and it must act on recommendations from the NAS for an overhaul of its opioid approval and removal policies. The broad indication on opioid labels must be narrowed, and an explicit warning against long-term use and high-dose prescribing should be added. FDA Compliant Nutrition Labels | Food Lab - NUTRITIONAL … FDA-compliant nutrition labels help you avoid misleading statements and claims to assure your food label artwork conveys truthful messaging. Along with preventing avoidable customer injury and potential litigation, compliant food labeling supports customers who are searching for the best food products for their families.
13 Misleading Food Label Claims and How Not to Be Tricked Oct 20, 2021 · The Food and Drug Administration (FDA) provides guidelines for a variety of common food labels, including sugar-free. While the term suggests that products labeled this way would be completely free of sugar, they can actually contain up to 0.5 grams of sugar in a single serving size.




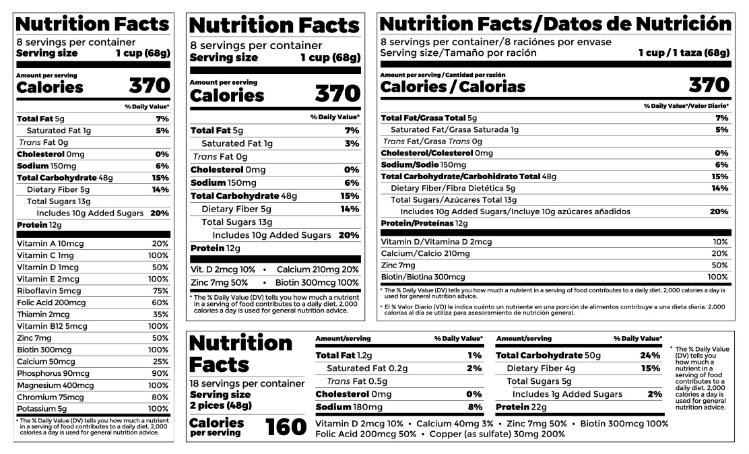



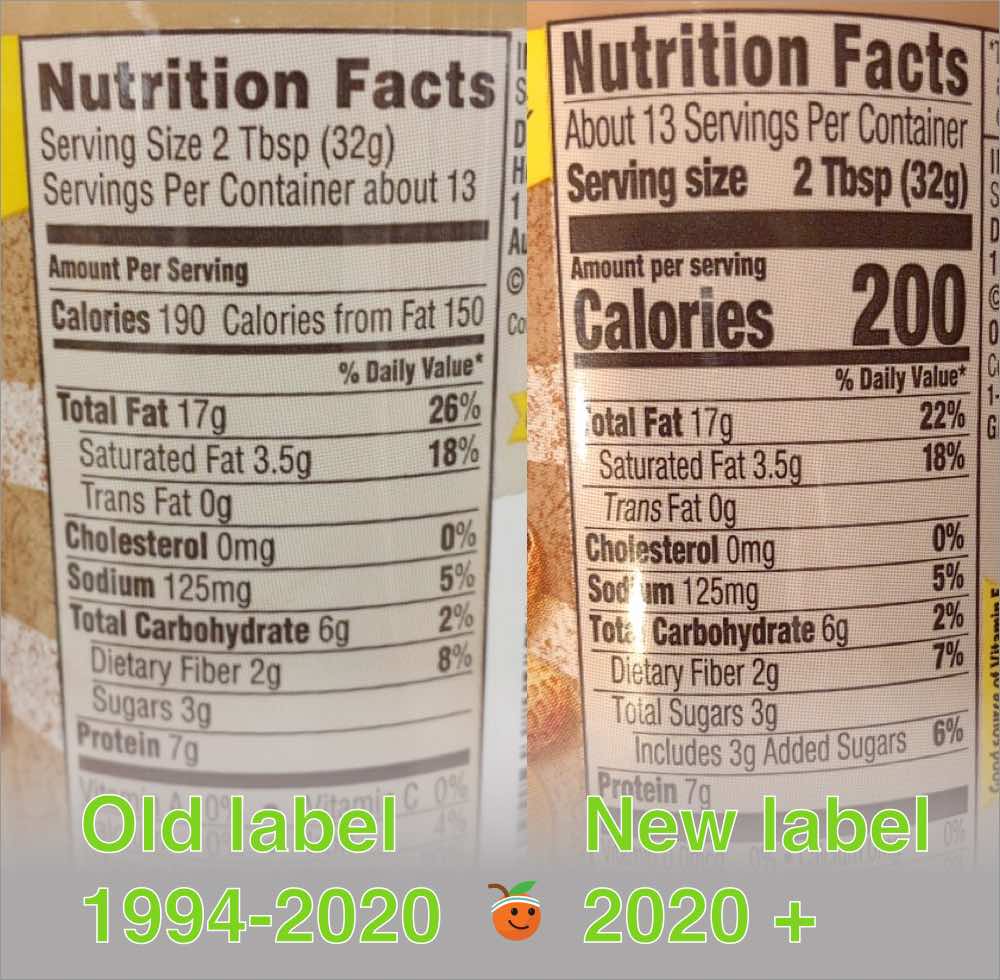



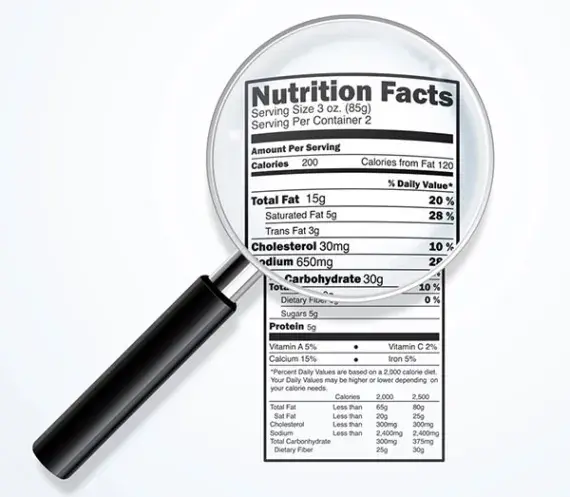






















Post a Comment for "44 fda misleading food labels"